CellR4 2013; 1 (3): e528
Pretransplant Infusion of Donor Stem Cells Open Gateway to Tolerance through Induction of Regulatory T Cells and Activation of Other Allosuppressive Immune Mechanisms – Single Centre Experience in Living Donor Renal Transplantation
Category: Original Articles
Abstract
PATIENTS AND METHODS: Ninety patients were subjected to pretransplant donor SCI of hematopoietic stem cells (HSC) and adipose tissue derived mesenchymal stem cells under non-myeloablative conditioning of Cyclophosphamide, rabbit-antithymocyte globulin, Rituximab and total lymphoid irradiation (TLI)/Bortezomib. Patients with diabetes, unwillingness, hepatitis C/B were excluded. Immune monitoring included donor specific antibodies (DSA) and peripheral regulatory T-cell (pTregs) [CD127low/-CD25highCD4+]. Initial maintenance immunosuppression was calcineurin-inhibitor based to be discontinued with stable graft function and absence of rejection episodes. Protocol biopsy was performed after 100 days of immunosuppression withdrawal in willing patients, for graft dysfunction. Rejections were treated by anti-rejection therapy followed by rescue immunosuppression.
RESULTS: All immunosuppression except Prednisone has been withdrawn for mean 2.8 years in 90 patients with mean age 32.2 years and donor-recipient HLA match, 2.81. Mean serum creatinine of 1.4 mg/dL and p-Tregs, 3.63% has remained stable after withdrawal. Rescue was required in 6 patients. DSA were absent in 37 patients and present in 53 patients. Protocol biopsies in all 26 willing patients were unremarkable.
CONCLUSIONS: This is the first clinical report showing induction of T-regs with SCI leading to stable graft function in LDRT on low dose steroid monotherapy. MSC may serve as novel, safe and effective immunomodulators in clinical transplantation.
Abbreviations
AD-MSC: adipose tissue derived mesenchymal stem cells; AR: acute rejection; BM: bone marrow; DSA: donor specific antibodies; HSC: hematopoietic stem cells; LDRT: living donor renal transplantation; pTregs: peripheral T regulatory cells; r-ATG: rabbit anti-thymocyte globulin; RT: renal transplantation; SCT: stem cell transplantation; SCr: serum creatinine.
Introduction
Transplantation is now a well accepted therapeutic option compared to dialysis for patients with end stage renal disease (ESRD). However, patients are required to be administered immunosuppressants life-long to prevent the graft rejection. Unfortunately these immunosuppressants have been able to control acute rejections partially; however, the problem of chronic rejections remains unanswered. In addition to causing financial burden to the individual/family/social system (depending upon the geographical location of the suffering patient) the recipient is prone to infections/malignancy which are detrimental to the graft and often responsible for causing morbidity and mortality. Thus transplanters have been caught in the vicious circle of their own success. The only answer to this problem is transplantation tolerance or transplantation with minimum/no immunosuppression rendering safe and meaningful healthy life to the transplant recipients. Tolerance may be defined as normal functioning graft in absence of immunosuppression while keeping third party immune response of the recipient intact. The true test for establishment of clinical tolerance in the transplant setting, the complete and successful withdrawal of immunosuppressive medications, has been achieved anecdotally and experimentally in rare renal transplant recipients1. Although such withdrawal was not planned in most cases, a number of trials are currently underway that include withdrawal of immunosuppression as part of the protocol 1 , 2 , 3. Salvateirra et al showed that DST can improve the renal graft survival 4. Taking lessons from this work, we started using stem cells (SC) to achieve transplantation with minimum/no immunosuppression 5. In furtherance of our objective; we designed a prospective trial to minimize immunosuppression to monotherapy of Prednisone, 5-10 mg/day in a cohort of willing living donor renal transplant (LDRT) recipients who had undergone pre-transplant SC therapy (SCT).
Patients and Methods
Ninety patients (81 males, 9 females) with mean age 32.2 ± 10.9 years and donor-recipient HLA match, 2.81 ± 1.33, were subjected to immunosuppression minimization. There were 5 patients with 0/6 HLA match, 10 patients with 1/6 match, 15 patients with 2/6 match, 38 patients with 3/6 match, 14 patients with 4/6 match, 5 patients with 5/6 match and 3 patients with full (6/6) HLA match. Donors were parents for 50 patients, spouses for 16, siblings for 16, children for 2 patients and unrelated (cross-over) in 6 patients. Original disease causing renal failure was unknown in 53 patients, hypertensive nephropathy in 11, chronic tubulointerstitial nephritis in 8, reflux nephropathy in 6, obstructive uropathy in 3, vasculitis in 2, Alport’s syndrome in 2, lupus nephritis in 2 and autosomal dominant polycystic kidney disease, focal segmental glomerulosclerosis and membranous nephropathy in 1 each.
All these patients underwent transplantation using pre-transplant SCT between September ’98 and November ’11. Inclusion criteria were stable graft function for ≥ 1 year with SCr < 2.0 mg/dL and absence of rejections. Unwilling patients, diabetics and hepatitis C/B were excluded from the trial because we believe that immunological status of these patients is different from others and we have separate protocols for such patients.
All patients received donor hematopoietic stem cells (HSCT) (mean, 71.3 ± 24.14 ml, with 0.26 ± 0.16 x 108 nucleated cells/kgBW of recipient with mean CD34+ count, 0.18 ± 0.22 x 106/kgBW). In addition donor adipose tissue derived mesenchymal stem cells (AD-MSC) (total 1.98 ± 0.97 x104 cells/kgBW) with mean CD45-/90+ cells, 0.64 ± 0.8 x 104/kgBW and CD45-/73+ cells, 0.24 ± 0.34 x 104/kgBW) were infused in 68 patients. Non-myeloablative conditioning of Cyclophosphamide, 20 mg/kgBW, rabbit-antithymocyte globulin (r-ATG), 1.5 mg/kg BW and Rituximab, 375 mg/m2 was given to all patients. In addition 42 patients had undergone total lymphoid irradiation (TLI), (200 CgY x 5) as a part of conditioning and 39 patients received Bortezomib, 1.3 mg/m2 x 4 along with methylprednisone, 125 mg intravenously instead of TLI prior to transplantation. (Bortezomib became available later on, hence it was replaced by TLI). Donor specific antibodies (DSA) (luminex single antigen assay) and Peripheral T-regulatory cells (p-Tregs) (CD127low/-/CD4+/CD25high) (flow cytometry) were tested before minimization of immunosuppression and three months after withdrawal of principal immunosuppressants.
DSA Measurement
All Class I and Class II antibody specificity screening was performed with Single Antigen Beads (One Lambda, Canoga Park, CA, USA). Screening tests for anti-HLA-specific IgG antibodies was performed using LABScreen® Single Antigen beads, class I and II (One Lambda Inc., Canoga Park, CA, USA). The assays were performed on Luminex platform following the manufacturer’s protocol. Trimmed mean fluorescence intensity (MFI) values were obtained from the output file generated by the flow analyzer, and normalized using the formula: [(Sample #N beads – Sample negative control (NC) beads) – (Negative control serum #N beads – Negative control serum NC beads)]. Any normalized MFI value over 2,000 were considered positive.
Measurement of pTregs
Measurement of pTregs was performed from peripheral blood of patients of all groups using CD127 mAb (PerCP-Cy), CD4 mAb (phycoerythrin [PE]), and CD25 mAb (fluorescein isothiocyanate [FITC]) [Becton Dickinson (BD) Biosciences, USA] according to manufacturer’s protocol using FACScan (BD Biosciences, USA).
Procurement of Peripheral Blood Stem Cells (PBSC)
PBSC was collected from cytokine stimulated donors for 2 days. They were subjected to leucopheresis on stem cell separator (Cobe Spectra version 7-Gambro, China). GCSF, 300 microgram, twice a day for 2 days, was used as cytokine for BM stimulation and mobilization before procurement.
Bone marrow (BM) aspiration and processing procedure
A total quantity of 100 ml BM was aspirated from posterior superior iliac crest of donor under local anesthesia (LA) and sedation (if donor was apprehensive) after cytokine stimulation for 2 days (as mentioned above).
The marrow was collected in transfer medium and transferred for culturing to stem cell lab to increase the yield of CD34+ cells by in vitro expansion and fortifying un-fractionated BM with stromal cells.
Portal infusion of stem cells
Under general anesthesia, a midline incision of approximately 3-5 cm length was made above the umbilicus by laparatomy, omental vein was identified and canulated with 20 guaze intracath. Stem cell bag was connected and they were infused directly without using any filters, at the rate of 6-8 ml/min. After infusion, omental vein was ligated with silk and hemostasis was checked. The wound was closed with vicryl 2/0, and subcuticular stitches were taken using 3/0 monocryl.
Stem cell lab protocols
In vitro expansion of HSC
BM collected from donor was transferred in self designed medium composed of Dulbecco’s Modified Eagle’s Medium (DMEM) with antibiotics and then immediately shifted to culture medium composed of DMEM with high glucose, essential amino acids, albumin, growth factors and antibiotics. Medium was replenished every other day for 8-10 days, the supernatant was removed on 7th/8th day and cultured marrow was mixed with AD-MSC after testing for viability, sterility, staining and quantification.
In vitro generation of AD-MSC
Adipose tissue was resected from anterior abdominal wall of kidney donor under LA after making a small incision on left lateral side below umbilicus. Sutures were taken after hemostasis was secured. This adipose tissue collected in self designed medium containing α-MEM, 20% human albumin and antibiotics, and was taken to the lab and minced with knife into tiny pieces. Then it was transferred in to the above medium with addition of collagenase type I, incubated at 37°C for 1 hour on self-designed shaker at 35-40 RPM for digestion. The contents of the medium processed in Petri dish were transferred to centrifuge tubes, centrifuged at 780 RPM for 8 minutes. The supernatant and pellets were separately cultured in the medium with same composition on 100 sq. cm and 25 sq. cm cell+ culture dishes (Sarsted, USA) respectively, at 37°C with 5% CO2 for 8-10 days. The medium was replenished every other day and then harvested by trypsinization. Collected cells after being tested were subjected to flow cytometric analysis. Cells were mixed with cultured BM and infused in portal circulation of patients.
Cell counts, viability and sterility
Total nucleated cell counts, viability and sterility tests were performed by standard lab techniques. SC were analysed using FACScan (BD Biosciences, US). CD34+/CD45+/CD33+/- cell lines were measured using CD33 mAb (PE-conjugated), CD34 mAb (FITC-conjugated) and CD45 mAb (PerCP-conjugated) (B.D. Biosciences, USA). For AD-MSC, CD 45-/90+ and CD 73+, CD 73 mAb (PE-conjugated), CD90 mAb (FITC-conjugated) and CD45 mAb (PerCP-conjugated) were used.
Peripheral blood and BM samples were collected in EDTA. In FACS tubes 20 µl of appropriate antibody was taken and 100 µl of blood was added. After vortexing for 5 seconds, the tubes were incubated in dark for 30 minutes. Then 2 ml of 10x lysing solution was added followed by centrifuging at 1000 RPM for 5 minutes. Supernatant was discarded and 2 ml of sheath fluid was added. The tubes were again centrifuged at 1000 RPM x 5 minutes. Supernatant was discarded. Finally 500 µl of sheath fluid was added for blood/AD-MSC samples and 1 ml was added for BM samples and subjected to data acquisition. Unstained blood samples were used as negative controls.
Initial maintenance immunosuppression consisted of calcineurin inhibitor (CNI) [Cyclosporin (3 mg/kgBW/day)/Tacrolimus (0.05 mg/kgBW/day)] or Sirolimus (1-2 mg/day) and/or mycofenolate sodium (360 mg BD)/Azathioprine (50-100 mg/day) and Prednisone (5-10 mg/day).
CNI levels and Sirolimus were measured at weekly intervals for first 2 months, fortnightly for the next 2 months and subsequently whenever indicated clinically, using Siemens reagent flex kit (Siemens RxL Max) according to manufacturer’s protocol with the aim of maintaining trough levels of CsA between 100-150 ng/ml and that of Tacrolimus and Sirolimus between 4-7 ng/ml. Mycofenolate measurement was not performed. Immunosuppression withdrawal was started with CNI followed by anti-proliferative agents. Prednisone was continued. Rejections were planned to be treated by anti-rejection therapy followed by rescue immunosuppression. Protocol biopsies were planned after 100 days of steroid monotherapy whenever patients gave their written informed consent. Trials were approved by the Institutional Review Board.
Results
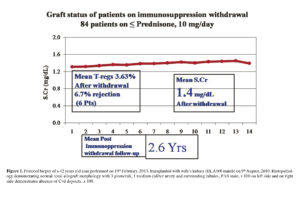
All immunosuppression except prednisone has been withdrawn in all 90 patients. Totally 6 (6.7%) patients developed acute rejections after mean 11.13 ± 5.18 months withdrawal and hence were rescued with mycofenolate. Their mean SCr of 1.71 ± 0.28 mg/dL has increased to 1.85 ± 0.53 mg/dL. Mean posttransplant follow-up is 6.23 ± 2.98 years and mean follow-up since steroid monotherapy is 2.8 ± 1.85 years. DSA-class-1 were present in 25 (27.8%), class-2 in 12 (13.3%), both in 16 (17.8%) and both were absent in 37 (41.1%) patients. Interestingly the same status remained even with steroid monotherapy. Protocol biopsies performed in all 26 patients who gave their consent, were unremarkable (Figure 1). Mean p-Tregs, 3.63 ± 1.55% and mean SCr of 1.4 ± 0.24 mg/dL at the time of immunosuppression withdrawal has remained at that level (Figure 2).
Discussion
At least three major mechanisms for tolerance induction have been proposed: clonal deletion, clonal anergy, and regulation/suppression 6 , 7 , 8 , 9. Most experts in the field agree that any durable tolerogenic therapy will involve manipulation of more than one mechanism, with the goal of profound reduction in clonal T-cell expansion accompanied by active immune regulation 10. Multiple receptors, ligands, and signaling intermediates have been identified that are instrumental to these processes and now serve as therapeutic targets for tolerance induction strategies, including co-stimulatory blockade, T-cell receptor targeting, and profound T-cell depletion. Extensive animal and human data suggest that the administration of donor antigen concurrent with these immunomodulatory agents may be an important adjunctive therapy for the success of any clinical tolerance strategies. With the understanding of these concepts, anecdotal reports have been published on tolerance induction however no definite reproducible model of deliberately induced and sustained clinical tolerance has evolved 11, 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21.
Our journey to the promised land of tolerance began in 1998 with megadose of HSC5.We kept on modifying our tolerance induction protocols with improvement in our understanding 22. The reason for using peripheral blood stem cells (PBSC) was that PBSC is chiefly composed of donor T-cells along with a small dose of CD34+ SC 23 , 24. Double negative (CD3- CD4- CD8-) putative T-regs in PBSC may counteract anti-donor T-cells, both systematically and locally as well as infiltrate the graft, thereby facilitating graft and donor cell survival 25 , 26. Portal infusion of SC was planned because liver is the most tolerogenic organ. It helps in achieving prope’ tolerance with low-grade lymphohematopoietic chimerism 27 , 28. We have observed contrary to the current understanding about thymic attrition with advancement in age, that thymus remains active in controlling central tolerance, and to be more definite about the SC reaching thymus, we directly injected them in to the thymus which preserves substantial numbers of T-regs in the recipient lymphoid repertoire as well as seeking apoptosis of activated T cells 29 , 30. We added nonmyeloablative conditioning treatment to create space in the marrow and reticuloendothelial systems, using cyclophosphamide and rabbit antithymocyte globulin to delete stimulated T-cell clones and rituximab to delete stimulated B-cell clones and control antibody response 31. However, our patients still required conventional immunosuppression. Hence we modified the regimen by adding irradiation 32 , 33. Radio-resistant NKT (natural killer T-cells) cells were thus able to interact with antigen presenting cells. At one stage we omitted thymic inoculation, which was deemed not acceptable. At this stage, we began to culture BM to generate a larger yield of CD34+SC. Subsequently we stimulated major histocompatibility complex (MHC)-restricted T- and B-cell clones and then deleted them. We then developed our own technique of generating human adipose tissue-derived mesenchymal stem cells (ad-MSC) in the lab and we fortified our SC with AD-MSC and HSC 34 , 35. By that time proteasome inhibitor bortezomib became available to delete plasma cells hence we replaced it with TLI 36 , 37. However with long experience we have realised that TLI before transplantation and Bortezomib after organ transplantation is still the most effective and safe conditioning.
MSC have immunomodulatory, immunosuppressive, and tolerogenic effects and also enhance the action of HSC 38 , 39. T-regs are involved in maintenance of tolerance and also dampen immune responses against cancer and allogeneic organs. MSC have been considered as a potential “homeostatic niche” for Tregs and play role in Treg recruitment and regulation and maintenance in vitro 40 , 41 , 42. Immunoregulatory functions of MSC are not fixed but rather the result of microenvironment they encounter in vivo 43. We believe that p-Tregs have protected the grafts of our patients from chronic rejections. SC, especially MSCs exhibit their genetically unrestricted immunosuppressive effects by inhibition of proliferation and function of T-cells, B-cells and NK cells in dose-dependent manner. MSCs also have tolerogenic effect by which they prolong survival of organ grafts and prevent graft versus host disease. MSCs avoid allogenic rejection by being hypoimmunogenic, modulating T-cell phenotype and by creating an immunosuppressive local milieu. Thus MSC exhibit immunogenicity, “tolerogenicity”, and immunosuppressive effects 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52. Control of chronic rejection which by and large occurs through the indirect pathway has been achieved in our model with sustained presence of p-Tregs.
Shortcomings of the present study
This study shows promising clinical results in achieving successful minimization of immunosuppression in LDRT to low dose steroid monotherapy. In this study we have not carried out chimerism studies since our previous experience indicated that peripheral blood chimerism may not be associated with absence of rejection episodes and vice versa 53 , 54. Protocol biopsies are also performed in limited number of patients since it is not easy to procure consent from patients. Financial constraints were the major shortcomings for performing more frequent immunologic monitoring. However we have already started performing DSA at 3 monthly intervals. Multi-centre trial will prove the beneficial effects mentioned here. However the major problem could be in replicating the in-vitro generation of adipose tissue derived MSC and a protocol which may require longer hospital stay.
In conclusion, we have achieved successful minimization of immunosuppression to low dose steroid monotherapy in LDRT using pre-transplant SCT with generation and recruitment of p-Tregs (CD127low/-/CD4+/CD25high).
Acknowledgements
The authors thank staff and technicians of IKDRC-ITS, India for all the technical help. We also thank Priyadarshini Shah, Shobhna Sengunthar and Yazdi Wadia from IKDRC-ITS who have maintained and provided the data on all the patient charts, follow-up and statistical analysis.
References
- Anderson D, Bilingham RE, Lampkin GH, et al. The use of skin grafting to distinguish between monzygotic and dizygotic twins in cattle. Heredity 1951; 5: 379-397. (back)
- Billingham RE, Brent L, Medawar PB. Actively acquired tolerance to foreign cells. Nature 1953; 172: 603-606. (back)
- Zukoski CF, Lee HM, Hume DM. The prolongation of functional survival of canine renal homografts by 6-mercaptopurine. Surg. Forum 1960; 11: 470-472. (back)
- Salvatierra O, Vincenti F, Amend W, et al. Four years experience with donor-specific blood transfusions. Transplant Proc 1983; 15: 924-931. (back)
- Trivedi HL, Shah PR, Shah VR, et al. High Dose DBMC Associated Tolerance in live related renal allograft recipients. Transplant Proceedings 2000; 32: 2001-2002. (back)
- Salama AD, Remuzzi G, Harmon WE, Sayegh MH. Challenges to achieving clinical transplantation tolerance. J Clin Invest 2001; 108: 943-948. (back)
- Salama AD, Womer KL, Sayegh MH. Clinical Transplantation Tolerance. Many Rivers to Cross. J Immunol 2007; 178: 5419-5423. (back)
- Matthews JB, Ramos E, Bluestone JA. Clinical trials of transplant tolerance: slow but steady progress. Am J Transplant 2003; 3: 794-803. (back)
- Dong VM, Womer KL, Sayegh MH. Transplantation tolerance: the concept and its applicability. Pediatr Transplant 1999; 3: 181-192. (back)
- Calne RY. The rejection of renal homografts. Inhibition in dogs by using 6-meracaptopurine. Lancet 1960; 1: 417-418. (back)
- Strober S, Dhillon M, Schubert M, Holm B, et al. Acquired immune tolerance to cadaveric renal allografts (A study of three patients treated with total lymphoid irradiation). N Engl J Med 1989; 321: 28-33. (back)
- Spitzer TR, Delmonico F, Tolkoff-Rubin N, McAfee S, Sackstein R, Saidman S, Colby C, Sykes M, Sachs DH, Cosimi AB. Combined histocompatibility leukocyte antigen-matched donor bone marrow and renal transplantation for multiple myeloma with end stage renal disease: the induction of allograft tolerance through mixed lymphohematopoietic chimerism. Transplantation 1999; 68: 480-484. (back)
- Burlingham WJ, Grailer AP, Fechner JH, et al. Microchimerism linked to cytotoxic T lymphocyte functional unresponsiveness (clonal anergy) in a tolerant renal transplant recipient. Transplantation 1995; 59: 1147-1155. (back)
- Sánchez-Fueyo A, Strom TB. Immunological tolerance and liver transplantation. J Hepatol 2004; 41: 698-705.]] , ((Mazariegos GV, Reyes J, Marino IR, et al. Weaning of immunosuppression in liver transplant recipients. Transplantation 1997; 63: 243-249. (back)
- Devlin J, Doherty D, Thomson L, Wong T, Donaldson P, Portmann B, Williams R. Defining the outcome of immunosuppression withdrawal after liver transplantation. Hepatology 1998; 27: 926-933. (back)
- Takatsuki M, Uemoto S, Inomata Y, et al. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation 2001; 72: 449-454. (back)
- Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355: 1318-1330. (back)
- Fudaba Y, Spitzer TR, Shaffer J, et al. Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: in vivo and in vitro analyses. Am J Transplant 2006; 6: 2121-2133. (back)
- Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 2008; 358: 353-361. (back)
- Brouard S, Mansfield E, Braud C, et al. Identification of a peripheral blood transcriptional biomarker panel associated with operational renal allograft tolerance. Proc Natl Acad Sci U S A 2007; 104: 15448-15453. (back)
- Martínez-Llordella M, Lozano JJ, Puig-Pey I, Orlando G, et al. Using transcriptional profiling to develop a diagnostic test of operational tolerance in liver transplant recipients. J Clin Invest 2008; 118: 2845-2857. (back)
- Vanikar AV, Goplani KR, A. Feroz, et al. Operational Tolerance in Living-Related Renal Transplantation: A Single-Center Experience. Transplant Proc 2011; 43: 1551-1558. (back)
- Trivedi H, Shah VR, Shah PR, et al. High dose DBMC associated tolerance in live-related renal allograft recipients. Transplant Proc 2000; 32: 2001-2002. (back)
- Trivedi H, Shah VR, Vanikar AV, et al. High-dose peripheral blood stem cell infusion: a strategy to induce donor-specific hyporesponsiveness to allografts in pediatric renal transplant recipients. Pediatric Transplantation 2002; 6: 63-68. (back)
- Bachar-Lustig E, Rachamim N, Li HW, et al. Megadose of T cell-depleted bone marrow overcomes MHC barriers in sublethally irradiated mice. Nat Med 1995; 1: 1268-1273. (back)
- Ford MS, Young KJ, Zhang Z, et al. The immune regulatory function of lymphoproliferative double negative T cells in vitro and in vivo. J Exp Med 2002; 2: 261-267. (back)
- Calne RY, Friend PJ, Moffatt S, et al. Prope tolerance, perioperative Campath 1H, and low dose cyclosporine monotherapy in renal allograft recipients (letter). Lancet 1998; 351: 1701-1702. (back)
- Trivedi H, Vanikar AV, Modi PR, et al. In pursuit of the ultimate: the initial Ahmedabad journey toward transplantation tolerance. Transplant Proc 2007; 39: 653-657. (back)
- Remuzzi G, Perico N, Carpenter C, et al. The thymic way to transplantation tolerance. J Am Soc Nephrol 1995; 5: 1639-1646. (back)
- Posselt AM, Barker CF, Tomaszewski JE, et al. Induction of donor-specific unresponsiveness by intrathymic islet transplantation. Science 1990; 249: 1293. (back)
- Turvey SE, Fry JW, Wood KJ. New insights on the mechanisms of acquired intrathymic tolerance. Curr Opin Organ Transplant 1999; 4: 50. (back)
- Spitzer TR. Non-myeloablative allogeneic stem cell transplant strategies and the role of mixed chimerism. Oncologist 2000; 5: 215-223. (back)
- Slavin, Slavin Z, Kaplan HS, et al. Transplantation of allogeneic bone marrow without graft-versus-host disease using total lymphoid irradiation. J Exp Med 1978; 147: 963-972. (back)
- Schaffler A, Buchler C. Concise review: adipose tissue derived stroma cells–basic and clinical implications for novel cell-based therapies. Stem Cells 2007; 25: 818-827. (back)
- Le Blanc K, Ringdén O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2003; 11: 321-334. (back)
- Finn PW, Stone JR, Boothby MR, et al. Inhibition of NFK-B dependent T-cell activation abrogates acute allograft rejection. J Immunol 2001; 167: 5994-6001. (back)
- Everly MJ, Everly JJ, Susskind B, et al. Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation 2008; 86: 1754-1761. (back)
- Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002; 30: 42-48. (back)
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105: 1815-1822. (back)
- Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002; 30: 42-48. (back)
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105: 1815-1822. (back)
- Koc ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after oinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 2000; 18: 307-316. (back)
- Casiraghi F, Perico N, Remuzzi G. Mesenchymal stromal cells to promote solid organ transplantation tolerance. Curr Opin Organ Transplant 2013; 18: 51-58. (back)
- Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002; 30: 42-48. (back)
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105: 1815-1822. (back)
- Koc ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after oinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 2000; 18: 307-316. (back)
- Casiraghi F, Perico N, Remuzzi G. Mesenchymal stromal cells to promote solid organ transplantation tolerance. Curr Opin Organ Transplant 2013; 18: 51-58. (back)
- Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 2003; 75: 389-397. (back)
- Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 2005; 105: 2214-2219. (back)
- Jiang XX, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005; 105: 4120-4126. (back)
- Prevosto C, Zancolli M, Canevali P, Zocchi MR, Poggi A, et al. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica 2007; 92: 881-888. (back)
- Maccario R, Podestà M, Moretta A, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica 2005; 90: 516-525. (back)
- Spitzer TR. Non-myeloablative allogeneic stem cell transplant strategies and the role of mixed chimerism. Oncologist 2000; 5: 215-223. (back)
- Vanikar AV, Trivedi HL, Feroze A, et al. Effect of co-transplantation of mesenchymal stem cells and hematopoietic stem cells as compared to hematopoietic stem cell transplantation alone in renal transplantation to achieve donor hypo-responsiveness. Int Urol Nephrol 2011; 43: 225-232. (back)
To cite this article
Pretransplant Infusion of Donor Stem Cells Open Gateway to Tolerance through Induction of Regulatory T Cells and Activation of Other Allosuppressive Immune Mechanisms – Single Centre Experience in Living Donor Renal Transplantation
CellR4 2013; 1 (3): e528
Publication History
Published online: 20 Nov 2013

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.