CellR4 2014; 2 (2): e839
Fashioning Cellular Rhythms with Magnetic Energy and Sound Vibration: a New Perspective for Regenerative Medicine
Topic: Regenerative Medicine
Category: Reviews
Abstract
Compelling evidence recently shows that oscillations and synchronization of multiple oscillators is an essential requisite in living cells. This review discusses the most update and intriguing investigations demonstrating that circadian clocks exist at the subcellular and single cell level. Chromatin remodeling and the orchestration of wide-ranging gene and protein expression profiles are fashioned into rhythmic synchronous oscillatory domains, capable to drive stem cell growth and differentiation, as well as the overall embryo development. The cytoskeleton has been shown to behave as a rhythmically oscillating network, generating radioelectric fields that may translate locally generated events into non-local long-range signaling. Worthy to note, aberrant oscillatory patterns are invariantly associated with various diseases. Consonant with a major role of physical forces in the specification of living process is the possibility to use physical energies to modulate (stem) cell homeostasis. We discuss recent findings showing that proper delivery of radioelectric fields is able to finely tune the expression of multipotency in human adult stem cells and to afford a direct reprogramming of human dermal skin fibroblasts into cardiac-, neuronal- and skeletal muscle-like cells. Electromagnetic energy was also found to efficiently counteract stem cell aging in vitro, restoring the multilineage potential of human stem cells throughout multiple passage in culture. We dissect the role of vibrational/acoustic energy as an inherent property of living cells, discussing the possibility to extract nano-mechanical signatures of stem cell differentiation that can be used to direct stem cell fate. On the whole, these new discoveries prompt a paradigm shift towards a deeper understanding of the interconnections between the physical Universe and the living World in the attempt to further approach the information of life.
Introduction
Oscillation and synchronization of multiple oscillators is a fascinating manifestation of self- organization in the Universe. There is now recent and compelling evidence that celestial mechanics is mirrored in molecular biology. Coupling of oscillatory patterns, and their synchronization into a rhythm is emerging as the underpinning of essential processes of life. Until recently, the standard view has conceived the mammalian circadian clock as a kind of orchestrator located at the level of suprachiasmatic nuclei and spreading through the living organism as a hierarchically organized system.
However, it is emerging that the circadian rhythm has its origin at the single cell level. Recent studies based on the use of single-cell techniques indicate that the rhythms in peripheral tissues are self-sustained networks of oscillations fashioned at single cell level, and rapid progress has been made in unraveling the molecular component of the clock, albeit with a still incomplete picture. Nevertheless, it is clear that any disturbance in the rhythm and resonance mode interaction of subcellular oscillators results in remarkable alteration in cellular dynamics, and abnormal behavior at the level of tissues and organs, implying a diseased status in the entire individual. Therefore, Physics is embedded in the living world and cellular oscillations cannot be viewed as mechanistic aggregates of multiple rhythms but as integral, intrinsically dynamic entities connected throughout space and time.
In the current review, we recapitulate the most updated evidence for such a view and summarize our recent findings indicating that magnetic energy and sound vibration can deeply impact onto a coordinated program of gene expression and signaling networks remarkably enhancing the expression of multipotency in embryonic and human adult stem cells, and counteracting stem cell aging and loss of differentiating potential due to geroconversion.
We will also report on the possibility of using a magnetic energy to reprogram human adult non-stem somatic cells to an embryonic-like pluripotent state, ensuing into lineages in which these cells would never otherwise appear, including cardiac, neural and skeletal muscle cells.
We will discuss the finding that acoustic vibrations are produced at a single cell level, and that cellular mechanics may represent a vibrational signature of cell health and differentiating potential.
We will highlight that many pieces of the puzzle are still to be unraveled, and that a new paradigm is necessary in Science and Medicine to avoid the crucial discrepancy that we are currently facing in the modern Science: due to the fragmented analysis of the living world, the more deep insight it proceeds into the mystery of life the farther it moves from the life itself.
Circadian Clocks Exist at Single Cell Level and Synchronize in Timely and Spatially Self-Organized Processes of Life
Circadian biological clocks are self-sustained oscillators that have been detected even at subcellular level. It has been shown that a clock transcription feedback loop generates cycles of NAD+ biosynthesis, ATP production, and mitochondrial respiration through modulation of mitochondrial protein acetylation to synchronize oxidative metabolic pathways with the 24-hour fasting and feeding cycle 1. Circadian control of the activity of the NAD+-dependent deacetylase sirtuin 3 (SIRT3) generated rhythms in the acetylation and activity of oxidative enzymes and respiration in isolated mitochondria, and NAD+ supplementation restored protein deacetylation and enhanced oxygen consumption in circadian mutant mice 2. These data indicate that circadian control of NAD+ bioavailability modulates mitochondrial oxidative function and organismal metabolism across the daily cycles of fasting and feeding.
Calcium ions (Ca2+) are crucial messenger of cellular homeostasis and fate in a wide variety of contexts, including lineage commitment in stem cells, the specification of developmental patterning in the embryo, neuronal plasticity and cell memory. Rather than changing in a discrete fashion (i.e increasing from a baseline to a stable long-lasting plateau and then declining again), cytosolic Ca2+ homeostasis is orchestrated in waves generating oscillatory Ca2+ signals. Experimental studies have shown that at single cell level, under certain conditions, initiation of Ca2+ waves is random in space and time, while under other conditions, waves occur repetitively from preferred locations (pacemaker sites) from which they entrain the whole cell. In both ventricular myocyte experiments and computer simulations of a heterogeneous Ca2+ release unit (CRU) network model, Ca2+ waves were found to occur randomly in space and time when the Ca2+ level is low, but as the Ca2+ level increases, waves occurred repetitively from the same sites 3. This transition to entrainment can be attributed to the fact that random Ca2+ sparks self-organize into Ca2+ oscillations differently at low and high Ca2+ levels. At low Ca2+, the whole cell Ca2+ oscillation frequency of the coupled CRU system is much slower than that of an isolated single CRU 4. Compared to a single CRU, the distribution of inter spike intervals (ISIs) of the coupled CRU network exhibits a greater variation, and its ISI distribution is asymmetric with respect to the peak, exhibiting a fat-tail 5. At high Ca2+, however, the coupled CRU network has a faster frequency and lesser ISI variation compared to an individual CRU. The ISI distribution of the coupled network no longer exhibits a fat-tail and is well-approximated by a Gaussian distribution. This same Ca2+ oscillation behavior was also found to be achieved upon variation in the number of ryanodine receptors per CRU or the distance between CRUs 6. These data, indicate that the process of entrainment of random oscillators can be modeled through theories that provide a unified explanation for the experimental observations underlying the emergence of pacemaker sites and rhythmic oscillations.
A critical issue in cellular oscillators is their precision and the implication(s) of such precision for circadian clocks. While the period of these oscillators evolved so that it resonates with the 24-h daily environmental cycles, the precision of the oscillator is another relevant property of cell-autonomous oscillators. Analysis of precision in cellular oscillators is of particular challenge. Novel theoretical schemes suggest that while the low-noise regime is faithfully recapitulated, increasing the level of noise leads to species-dependent precision 7. As noise increases, it appears that subcomponents of the oscillator gradually decouple from the core oscillator itself, which has allowed to identify the subnetworks responsible for robust rhythms 8.
The synchronization of coupled oscillators is a crucial issue in the orchestration of essential processes of life, such as the beating of the heart. Although it was long thought that synchrony and disorder were mutually exclusive steady states for a network of identical oscillators, numerous theoretical studies in recent years have revealed the intriguing possibility of “chimera states”, in which the symmetry of the oscillator population is broken into a synchronous part and an asynchronous part. However, empirical evidence for chimera states in natural systems has been lacking until very recently, when it has been shown that within mechanical oscillators coupled in a hierarchical network chimeras emerge naturally from a competition between two antagonistic synchronization patterns. To this end, Martens and Coworkers have recently implemented the simplest form of nonlocal coupling that can be achieved using a hierarchical network with two subpopulations: within each subpopulation, oscillators are coupled strongly, whereas the coupling strength between the two subpopulations is weaker 9. They placed N identical metronomes with a nominal beating frequency f on two swings, which can move freely in a plane. Oscillators within one population were coupled strongly by the motion of the swing onto which the metronomes are attached. As f is increased, more momentum is transferred to the swing, effectively leading to a stronger coupling among the metronomes. A single swing follows a phase transition from a disordered state to a synchronized state as the coupling within the population increases. In their mathematical model, the Authors show that self-organization was controlled by elementary dynamical equations from mechanics that are ubiquitous in many natural and technological systems 10. These findings are of particular relevance, providing evidence that symmetry-breaking mechanism may be a prevalent feature in systems exhibiting collective behavior, no matter whether they are power grids, optomechanical crystals, or living cells.
Within cellular oscillators, cell density plays an important role in governing the circadian rhythmicity. Single-cell imaging of fibroblasts from PER2::LUC circadian reporter mice revealed greatly reduced PER2::LUC rhythmicity in low-density cultures 11, which could result from lack of either constitutive or rhythmic paracrine signals from neighboring fibroblasts. To discriminate between these two possibilities, PER2::LUC wild-type (WT) cells were mixed with nonluminescent, nonrhythmic Bmal1-/- cells, so that density of rhythmic cells was low but overall cell density remained high. In this condition, WT cells showed clear rhythmicity similar to high-density cultures. Similar results were yielded when PER2::LUC WT cells were mixed with nonluminescent, long period Cry2-/- cells 12. Moreover, conditioned medium from high-density fibroblast cultures rescued rhythmicity of low-density cultures 13. On the whole, these data suggest that within a cell population normal expression of rhythmicity requires paracrine signals from adjacent cells, but that these signals do not have to be rhythmic, and that intrinsic periods on a given cell population are not affected by the rhythmic signals from other cells. These findings prompt further studies to elucidate the composition of the overall secreted proteins (secretome) involved in paracrine specification and maintenance of rhythmic signals, and further deployment of the resonance modes among rhythmic oscillators based on cell crowding.
Cellular Oscillators Orchestrate the Embryogenesis and the Differentiating Patterning in Stem Cells
Embryo development relies on the timely proliferation of progenitor cells and their subsequent differentiation into multiple lineages. Modulation and orchestration of the timing of cell differentiation and cell fate choice are key issues for making organs of the right size, shape and cell composition. In the entire organism, cell proliferation and differentiation are antagonistically regulated by multiple basic helix-loop-helix (bHLH) activator and repressor genes. In particular, the Hes bHLH repressor genes control essential developmental pathways by maintaining self-renewal of progenitor cells and regulating binary cell fate decisions 14. In the developing nervous system, stem cells proliferate and sequentially give rise to different cell types by modulating their differentiation potential. Without Hes genes such as Hes1, stem cells prematurely differentiate into certain types of neurons only, and are depleted before they have proliferated sufficiently to provide all neuronal and glial cell types. This results in small and deformed brain structures. Hes genes also function as biological clocks, measuring time in developmental events, such as somite segmentation. It has been shown that the expression of Hes1 and Hes7 oscillates with a periodicity of 2 hours. Hes1 regulates the timing of biological events in many cell types, such as fibroblasts, whereas Hes7 functions as the segmentation clock. Hes1 oscillation is cell-autonomous and relies on negative autoregulation. After induction, Hes1 protein represses the expression of its own gene by directly binding to its promoter. The short-living repression, due to the short half-life of the mRNA and protein, accounts for the observed autonomously initiated oscillatory pattern. This oscillation is transient and dampened after three to six cycles (6-12 hours). However, this behavior is not due to dampened oscillation within individual cells, in which Hes1 expression is still cyclical even after nearly 2 days, as revealed by real-time imaging analysis 15. Rather, the dampening arises because the oscillation period varies from cycle to cycle within a cell, and, therefore, oscillations among cells easily fall out of synchrony. This loss of synchrony explains why, after several cycles, the oscillatory expression of Hes1 is not detected by northern or western blot analysis. Similarly, even in stationary cultured cells where Hes1 levels seem to be constant by northern and western blot analyses, Hes1 expression is found to be dynamically changing at the single-cell level 16. Thus, at any given time, Hes1 expression levels are variable within and between cells, which may enable a cell to mediate a different response to the same stimulus. Hes1 oscillation may regulate the timing of cellular events, such as the cell cycle, but its precise roles are unknown. Within the Hes family of genes, Hes7 is an essential component of the segmentation clock. Somites, the segmental units that later give rise to the vertebrae, ribs, skeletal muscles and dermis, are formed by the segmentation of the anterior region of the presomitic mesoderm 17. This event is repeated every 2 hours in mouse embryos. It has been suggested that this periodic event is controlled by a biological clock, called the segmentation clock. It was first reported that the expression of chairy1, a chick homolog of mouse Hes1, is periodically propagated in a wave-like fashion initiating at the posterior end and moving towards the anterior region of the presomitic mesoderm (PSM) (propagation is classified into three phases) 18. Each wave leads to the generation of a pair of somites. In the mouse PSM, Hes1, Hes5 and Hes7 are expressed in a similar fashion to each other, but Hes7 is the most important for somite segmentation. This wave-like expression of Hes7 is elicited by its oscillatory expression in each PSM cell. One of its downstream target genes is the glycosyltransferase lunatic fringe (Lfng), which regulates Notch activity by glycosylation 19 , 20. Hes7 protein represses transcription from the Lfng promoter as well as from its own, and thus Lfng expression oscillates in phase with Hes7 expression 21. Lfng periodically inhibits Notch signaling and thereby generates oscillations in Notch activity 22 , 23 , 24, which may in turn influence Hes7 oscillation. It is thought that the combined effects of these coupled negative-feedback loops on Hes7 and Lfng expression are important for sustained and synchronized oscillations and for the correct timing of the biological clock.
Embryo development and lineage commitment in stem cells are also associated with the emergence of lineage-specific chromosomal topologies. Recent data provide evidence that chromatin remodeling, as it occurs during the differentiation of multipotent stem cells, involves cell-specific nuclear organization, and dramatic changes in total genome order that can be modeled using the mathematical approaches of distance matrices and coupled oscillators 25. Commitment of the progenitor cells results in an initial increase in entropy at both the level of gene coregulation and chromosomal organization, which may represent a phase transition, followed by a progressive decline in entropy during differentiation. The stabilization of a highly ordered state in the differentiated cell types results in lineage-specific chromosomal topologies and is related to the emergence of coherence-or self-organization-between chromosomal associations and coordinate gene regulation. These data suggest that even chromatin remodeling and coupling of changes in genome structure and function during (stem) cell commitment may imply complex dynamics of entangled of oscillatory patterns.
Synchronization of multiple oscillators at single stem cell level involves the temporal and spatial organization of cellular ion homeostasis and electric microcurrent in wavelet/wave-like fashion. A typical example of multifaceted syncronization among multiple oscillatory patterns is provided by cardiac myocytes. In this field, there is an intense interest in differentiating embryonic stem cells to engineer biological pacemakers as an alternative to electronic pacemakers for patients with cardiac pacemaker function deficiency 26. Deciphering the signaling events that lead to a timely assembly of cellular oscillators into functionally competent pacemaker cells would have obvious implications in the cell therapy of severe arrhythmias. Embryonic stem cell-derived cardiocytes (ESCs), however, often exhibit dysrhythmic excitations. Using Ca2+ imaging and patch-clamp techniques, the analysis of requirements for generation of spontaneous rhythmic action potentials (APs) in late-stage mouse ESCs revealed that the sarcoplasmic reticulum (SR) was able to generate spontaneous, rhythmic, wavelet-like Local Ca2+ Releases (LCRs) (inhibited by ryanodine, tetracaine, or thapsigargin). L-type Ca2+ current (I(CaL)) induced a global Ca2+ release (CICR), depleting the Ca2+ content SR which resets the phases of LCR oscillators. Following a delay, SR was found to generate a highly synchronized spontaneous Ca2+ release of multiple LCRs throughout the cell. The LCRs in turn generated an inward Na+/Ca2+ exchanger (NCX) current (absent in Na+-free solution) that ignites the next AP. Interfering with SR Ca2+ cycling (ryanodine, caffeine, thapsigargin, cyclopiazonic acid, BAPTA-AM), NCX (Na+-free solution), or I(CaL) (nifedipine) resulted in dysrhythmic excitations or cessation of automaticity. Inhibition of cAMP/PKA signaling by a specific PKA inhibitor, PKI, decreased SR Ca2+ loading, substantially reducing both spontaneous LCRs (number, size, and amplitude) and rhythmic AP firing. In contrast, enhancing PKA signaling by cAMP increased the LCRs (number, size, duration) and converts irregularly beating ESCs to rhythmic “pacemaker-like” cells. SR Ca2+ loading and LCR activity could be also increased with a selective activation of SR Ca2+ pumping by a phospholamban antibody.
On the whole, these data indicate that rhythmic behavior and oscillator synchronization may involve Ca2+ loading of intracellular Ca2+ pool(s) (SR in the cardiomyocytes) and spontaneous rhythmic LCRs in the form of wavelet-like signal driven by timely protein kinase(s) activity and kinase-second messenger interplay as that observed for cAMP/PKA activity in cardiomycoytes. Further entanglement may be provided by synchronization of multiple LCR oscillators by I(CaL), resulting in strong, partially synchronized diastolic Ca2+ release and NCX current. Rhythmic ESC automaticity can be therefore achieved by boosting “coupling” factors, such as cAMP/PKA signaling, that enhance interactions between SR and sarcolemma.
The impact of physical loading is a potent stimulus required to maintain (stem) cell homeostasis, but the mechanism (s) by which cells can sense a biophysical force and translate that into a developmental decision (mechanotransduction) remains poorly understood. The primary cilium is a single sensory cellular extension, which has recently been shown to demonstrate an important role in mechanotransduction and lineage commitment in human mesenchymal stem cells (hMSCs). It is intriguing that even this sort of cellular antenna has been fashioned to act as a sensor of oscillatory patterns 27. In fact, it has been shown that short periods of mechanical stimulation in the form of oscillatory fluid flow (OFF) is sufficient to enhance osteogenic gene expression and proliferation of human mesenchymal stem cells (hMSCs). Furthermore, the cilium mediates fluid flow mechanotransduction in hMSCs by maintaining OFF-induced increases in osteogenic gene expression and, surprisingly, to limit OFF-induced increases in proliferation.
The general relevance of the primary cilium as an oscillatory mechanosensor is underlined by its ability to coordinate early cardiogenesis and hedgehog signaling during cardiomyocyte differentiation. It has been shown that the pluripotent P19.CL6 mouse stem cell line, which can differentiate into beating cardiomyocytes, forms primary cilia that contain essential components of the hedgehog pathway, including Smoothened, Patched-1 and Gli2 28. Knockdown of the primary cilium by Ift88 and Ift20 siRNA or treatment with cyclopamine, an inhibitor of Smoothened, blocked hedgehog signaling, as well as differentiation into beating cardiomyocytes. Very recently, a tight interplay between surface contact topography within the cell culture environment and the structure and function of the primary cilium was found to play an essential role in the growth and differentiation of hMSCs 29.
These findings may have remarkable implication in the development of novel cell therapy strategies. In fact, the application of OFF period with defined oscillatory patterning coupled with targeted surface topographies may be beneficial components of bioreactor-based approaches to form stem cell-derived specific tissues suitable for regenerative medicine. Moreover the primary cilium may become itself a potential therapeutic target for efforts to mimic loading with the aim of optimizing stem cell commitment and differentiation.
Aberrant Oscillatory Patterning Invariantly Associates with Disease
In the absence of Hes7, somites fuse, which results in fused vertebrae and ribs 30. Interestingly, a lack of oscillation due to persistent Hes7 expression also leads to somite fusion 31. Hes7 oscillation, like that of Hes1, is regulated by negative feedback 32. In the absence of Hes7, Lfng is constitutively expressed in the PSM 33. Lfng oscillation is also crucial for segmentation, as both the loss of and persistent expression of Lfng has been shown to lead to severe somite fusion 34 , 35 , 36.
Defects in the assembly or rhythmic function of primary cilia, which are oscillatory sensory organelles, are tightly coupled to developmental defects and diseases in mammals. Primary ciliary dyskinesia most often arises from loss of the dynein motors that power ciliary beating. In this regard, it has been shown that DNAAF3 (also known as PF22), a previously uncharacterized protein, is essential for the preassembly of dyneins into complexes before their transport into cilia 37. Loss-of-function mutations in the human DNAAF3 gene were identified in individuals from families with situs inversus and defects in the assembly of inner and outer dynein arms 38. Knockdown of DNAAF3 in zebrafish likewise disrupts dynein arm assembly and ciliary motility, causing primary ciliary dyskinesia phenotypes that include hydrocephalus and laterality malformations 39. These results support the existence of a conserved, multistep pathway in the cytoplasmic assembly of competent ciliary dynein complexes.
E11.5 embryos of the Ift88(tm1Rpw) (Ift88-null) mice, which form no cilia, have ventricular dilation, decreased myocardial trabeculation and abnormal outflow tract development (Clement CA, Kristensen SG, Møllgård K, et al. The primary cilium coordinates early cardiogenesis and hedgehog signaling in cardiomyocyte differentiation. J Cell Sci 2009; 122(Pt 17): 3070-3382.). These data support the conclusion that cardiac primary cilia are crucial in early heart development, where they partly coordinate hedgehog signaling.
In general, malfunction of the circadian timing system may result in cardiovascular and metabolic diseases, and conversely, these diseases can impair the circadian system. Studies conducted in spontaneously hypertensive rats (SHR) revealed that SHR exhibited an early chronotype, since the central clock in the suprachiasmatic nuclei (SCN) was phase advanced relative to light/dark cycle and the SCN driven output rhythm ran faster compared to Wistar rats 40. Moreover, the output rhythm was dampened. The SHR peripheral clock reacted to the dampened SCN output with tissue-specific consequences. In the colon of SHR the clock function was severely altered, whereas the differences are only marginal in the liver 41. These changes may likely result in a mutual desynchrony of circadian oscillators within the circadian system of SHR, thereby potentially contributing to metabolic pathology of the strain. The SHR may thus serve as a valuable model of human circadian disorders originating in poor synchrony of the circadian system with external light/dark regime.
Driving Stem Cell Fate with Magnetic Energy and Sound Vibration
Effect of Electromagnetic Fields
As discussed above, oscillatory patterns and their synchronization into resonating rhythms identify dynamic contexts that transcend the boundary of a single cell level, extending to and specifying the long-range assembly of tissues, organs, and of the entire individual. There is compelling evidence that cytoskeleton, which forms the physical and biochemical interface for a large variety of cellular processes, plays an essential role in the orchestration of mechanical and signaling patterning of living cells. Microtubules are important structures in the cytoskeleton, and have long been shown to display intrinsic oscillatory patterns. Moreover, microtubules are electrically polar. Further deployment of microtubular oscillatory dynamics and polarity has recently placed the role of the cytoskeleton into a novel perspective. In fact, it is conceivable that certain microtubule normal vibration modes efficiently generate oscillating electric field. This oscillating field may be important for the intracellular organization and intercellular networking. Worthy to note there is strong experimental evidence which indicates electrodynamic activity of multiple cell types in the frequency region from kHz to GHz, expecting the microtubules to be the source of this activity 42 In a recent and comprehensive review, Zhao Y and Zhan Q 43 integrated a large body of studies reporting the existence of dielectrophoretic forces and electromagnetic interaction around and between cells in different experimental conditions, discussing how cellular dynamics may be regulated by electric fields generated by synchronized oscillations of microtubules, centrosomes and chromosomes. The intensities of electric field and of radiated electromagnetic power from the whole cellular microtubule network have been calculated 44. The subunits of microtubule (tubulin heterodimers) can be approximated by elementary electric dipoles. Mechanical oscillation of microtubule can be represented by the spatial function which modulates the dipole momentum of subunits. In these studies, the field around oscillating microtubules has been calculated as a vector superposition of contributions from all modulated elementary electric dipoles which comprise the cellular microtubule network 45. Theoretical analysis of the electromagnetic radiation and field characteristics of the whole cellular microtubule network indicate that a macroscopic detection system (antenna) is not suitable for measurement of cellular electrodynamic activity in the radiofrequency region since the radiation rate from single cells is very low (lower than 10-20W). Low noise nanoscopic detection methods with high spatial resolution which enable measurement in the cell vicinity are desirable in order to measure cellular electrodynamic activity reliably.
Nevertheless, these studies suggest the possibility that cell behavior and fate may be influenced by magnetic fields. In this regard, we have previously shown that exposure of mouse embryonic stem (ES) cells to extremely low frequency pulsed magnetic fields (50 Hz, 0.8 MTrsm) was able to enhance selectively the commitment to a cardiogeneic lineage, increasing the number of spontaneously beating, ES-derived cardiomyocytes 46. The effect was mediated by an increase in the transcription rate of a number of cardiogenic and cardiac-specific genes, involving the expression of their biologically active end-products 47. For decades Scientists have attempted to drive stem cell fate through the use of chemistry. These findings shown for the first time the possibility to use magnetic energy to drive stem cell growth and differentiation and lead to a number of interrelated considerations: (i) electromagnetic resonance modes between the endogenous cellular radioelectric (electromagnetic) oscillations are very likely occurring, although hardly detectable; (ii) every particular level of cellular hierarchy conceivably possesses a characteristic spectrum of endogenous electromagnetic oscillations originating from various processes; (iii) intra- and inter-level resonances should occur and provide a networking landscape between these processes. These considerations may harbor an important implication: modulation of these resonance modes may be conceived as a new strategy to handle (stem)cell homeostasis and fate. Accordingly, this may be achieved by appropriate delivery of radiofrequency magnetic fields to an entire organism or isolated cells, in conjunction with the establishment of a resonance circuit loop between the cellular microcurrents induced/modulated by the delivered magnetic field and the targeted organism (or cells) itself. We have recently accomplished this task by exposing isolated cells to a Radio Electric Asymmetric Conveyer (REAC), an innovative device based upon the interaction of two oscillating magnetic fields. One is generated by the entire organism or cultured cells, and the other is a weak (2 mW radiated power) electromagnetic field of 2.4 GHz produced by the REAC system. This interaction results in the induction/modulation of cellular electric microcurrents which are detected and conveyed back to a targeted area of the body or to isolated cultured cells by the aid of an electrode probe 48. We exposed mouse ES cells to REAC asymmetrically conveyed radioelectric fields of 2.4 GHz, using a REAC apparatus with its conveyer electrodes immersed into the culture medium 49. Cellular responses were investigated by real-time PCR, Western blot and confocal microscopy. Single radio frequency burst duration, radiated power, electric and magnetic fields, specific absorption rate, and current density in culture medium were monitored. REAC stimulation primed the transcription of genes involved in cardiac, skeletal muscle and neuronal commitment, also modulating the expression of the self renewal/pluripotency-associated genes 50. REAC exposure enhanced the expression of cardiac, skeletal and neuronal lineage-restricted marker proteins. The number of spontaneously beating ES-derived myocardial cells was also increased 51.
Based on these results, we exposed to REAC human mesenchymal stem cells isolated from the adipose tissue (hASCs) with a novel non-enzymatic method 52 and provided evidence that this treatment remarkably enhanced the transcription of a program of multilineage, tissue restricted genes 53, including: (i) the cardiogenic genes prodynorphin, GATA-4 and Nkx-2.5; (ii) the vasculogenic genes Vascular Endothelial Growth Factor (VEGF), Hepatocyte Growth Factor (HGF), and von Willebrand Factor (vWF); neurogenin-1, and myoD, involved in neurogenic and skeletal myogenic commitment, respectively. REAC exposure had no detrimental effects on hASCs, since it did not affect significantly the amount of apoptotic, or necrotic cells. Stem cell exposure to REAC also finely tuned the expression of stemness-related genes, inducing an early increase in Nanog, Sox-2 and Oct-4 transcription during the first 4-12 hours, followed by a significant down-regulation of transcript levels below the control value after 24 hours of treatment 54. Such a transcriptional profile was mirrored at the protein expression level. Confocal microscopy analysis shown the appearance of tissue specific markers for cardiogenic (a-sarcomeric actinin), neurogenic (β-3-tubulin), skeletal muscle (myoD), and endothelial (vWF) commitment in REAC-exposed hASCs, indicating that the induction of a tissue restricted program of gene and protein expression elicited by REAC converged to a lineage specification in intact cells 55. The finding that REAC exposure elicited a concomitant, early expression of both stemness-related and lineage-restricted genes together with a high-throughput of cellular differentiation indicate that its action may result from the optimization of stem cell expression of multipotency. In this regard, the downregulation in stemness gene and protein expression observed after 12 h of REAC exposure may be worthy of consideration, since it is now evident that the downregulation of Sox-2, Nanog and Oct-4, after their initial induction, is a critical step in cell progression towards a differentiated state 56 , 57 , 58 , 59. The outcomes of the synergistic interplay between REAC asymmetrically conveyed radio electric fields and hASCs were achieved after extremely brief exposure pulses, showing long-lasting persistence of cell commitment upon the cessation of REAC treatment. Ongoing studies will dissect the electrophysiological and functional properties of REAC-committed hASCs, and assess whether improved tissue healing may result from their transplantation in defined animal models of disease, including heart failure, neurodegeneration and skeletal muscle dystrophy. In the affirmative, the proper use of electromagnetic energy may represent the underpinning for future cell therapy approaches.
Recently, we shown that REAC conveyed radioelectric fields afforded a direct reprogramming of human dermal skin fibroblasts into cardiac, neuronal and skeletal muscle lineages 60. The effects were transcriptionally mediated, leading to the gene expression of a set of cardiogenic/neurogenic genes, including Mef2c, Tbx5, Gata4, and prodynorphin, as well as neurogenic and skeletal myogenic orchestrators, such as neurogenin1 and myod, respectively 61. Similar to the effects yielded in hASCs, REAC exposure of human fibroblasts induced a biphasic response on pluripotency genes, enhancing the expression of Nanog, Sox2, Oct4, and cMyc during the first 6-20 hours, while producing a stable downregulation in their transcription after 24 hours 62. For the first time, human non-stem somatic adult cells were rapidly committed to a high-yield of critical fates in Regenerative Medicine, only proceeding through a transient pluripotent state, without being plastered in such intermediate condition. This may avoid the risk of persistence of stray cells that haven’t fully differentiated and might have the ability to turn into an unwanted cell type, like cancer cells or cells that fail to fulfill the desired requirement(s) for tissue repair. Importantly, these results were achieved without the aid of potentially harmful viral or protein transduction. On the whole, REAC acted as a time machine capable to reset the clock of somatic human adult cells to an ancestral time where they may behave as pluripotent elements capable of multifaceted phenotypic decisions.
A major problem in the clinical application of cell therapy is the progressive decline of stem cell multipotency with age. Stem cell aging may not only impair the self-healing properties of tissue resident stem cells, but may also blunt the rescuing potential of stem cell transplantation in both autologous and allogenic settings, as the donor’s age increases. We have recently shown that geroconversion of hASCs and loss of their multilineage commitment after multiple passages in culture (a method to induce cell aging in vitro) were efficaciously counteracted by hASC exposure to REAC delivered radioelectric fields. In particular, REAC treatment afforded a marked downregulation in beta-galactosidase staining 63 , 64, and in the expression of the senescence mediator genes p16INK4, ARF, p53 and p21CIP1 65.Moreover, unlike unexposed cells, REAC treated hASCs maintained their typical fibroblast-like morphology, as well as a multilineage potential along osteogenic, adipogenic, chondrogenic, and vasculogenic fates, even at late passages, both at morphologic and gene expression levels.
So far, reversing of stem cell aging could only be achieved by the aid of viral vector-mediated gene delivery 66. For the first time, age-associated molecular and morphological alterations were reversed in stem cells by exposure to an innovative system of electromagnetic energy delivery, suggesting future perspectives in the clinical application of the REAC technology for the treatment of pathologic aging and degenerative diseases. Accordingly, we found that the REAC treatment was able to reestablish the normal cellular homeostasis in human chondrocytes obtained from the femoral head articular cartilage of patients with osteoarthritis (OA) 67, the most common articular degenerative disease of the hyaline cartilage and subchondral bone. Chondrocytes are responsible for the synthesis and maintenance of extracellular matrix within articular cartilage, balancing both synthetic and degradative processes. Exposing human OA chondrocytes to the REAC delivered radioelectric field antagonized the detrimental consequences of cell exposure to Interleukin-1b ( IL-1β) 68, a cytokine involved in cartilage degeneration, with a major impact on OA onset and progression through the secretion of pro-inflammatory cytokines, chemokines, neutral metalloproteinases (MMPs), and nitric oxide (NO). Other important effects of IL-1β in chodrocytes include the inhibition of proteoglycans (PGs) and collagen synthesis, and the induction of apoptosis. REAC downregulated both NO and MMP3 production in normal and OA human chondrocytes, while increasing PG synthesis 69. OA chondrocytes were more affected than normal chondrocytes by REAC treatment. The ability of REAC to counteract the degenerative action of IL-1β was confirmed by ultrastructural analysis, showing an increased number of mitochondria and Golgi bodies, along with a consistent reduction in the number of vacuolized cells, in REAC exposed OA chondrocytes 70.
Cell Reprogramming and Sound Vibration
The constitutive role of spontaneous and modulated oscillatory patterning in cellular homeostasis, the intrinsic oscillatory features of the cytoskeleton, and the ability of cells of fashioning their temporal and spatial chromatin organization through amplitude and frequency dependent tuning of their intrinsic oscillators, form a solid rationale for the deployment of (nano)mechanical vibrations as a strategy to reprogram cellular decisions and fate. On these bases, it is also becoming clear that mechanical dynamics and the mechanosensing apparatus are deeply involved in intra- and inter-cellular cross-talk and may be recruited to enhance the healing potential from tissue-resident stem cells. Mechanical boundary conditions drive transcriptional programs, homing, engraftment and fate of stem cells in living tissues, also providing relevant cues for the development of engineered tissues 71 , 72. Based on the different behavior of the mechanosensing apparatus in undifferentiated and terminally committed stem cells 73, innovative strategies for tissue repair may be envisioned through the modulation of stem cell mechanosensors. Stem cell engraftment after transplantation, an extremely low-yield process limiting tissue repair in animal models and patients 74, may also be rewardingly improved by the synergistic interplay between biochemical signals, including growth factors, and adhesion molecules similar to those engaged by leukocytes for recruitment to inflammatory sites 75 , 76 , 77. At the site of tissue damage, these forces may therefore orchestrate the secretion of autocrine/paracrine factors, significantly improving the homing and engraftment of circulating or transplanted stem cells, as suggested by the ability of cyclic stretch to act in cardiac fibroblasts enhancing the synthesis and secretion of insulin-like growth factor-1 (IGF-1), a peptide tightly involved in stem cell migration and homing 78. Mechanistically, physical forces can coordinate entailed signals between defined tissue elements, regulating stem cell growth and differentiation, especially in tissues chronically exposed to mechanical loading, such as the myocardium, and the vessel wall.
In vivo, stem cells reside within highly dynamic microenvironments, referred to as niches, where they receive complex anisotropic physical stimuli, occurring as time-scaled, as well as synchronous events. Within the niche, extracellular matrix displays hierarchical nanotopographies embedding stem cells with other non-stem elements 79: in this multifaceted domain, multiple signals self-organize as oscillatory patterns, undergoing synchronization and phase coherent organization which associate with remarkable structural and functional changes. This may be the context where (stem)cell experience and memory develop, creating the building blocks of the information needed to connect multipotency with tissue homeostasis and/or needs for repair.
Hierarchical assembly of nano-architechtonics through supra-molecular interactions will produce highly coordinated motions, as occurring in the continuous cytoskeletal remodeling, leading to changes in the local cell membrane Young’s modulus or viscoelastic properties. Stem cells can “sense” the extracellular and intracellular matrix as a dynamic niche, bearing information through the deployment of a number of motion-tenso-elastic features.
Within this context, we have demonstrated and patented for the first time the ability of cells to express “vibrational” (nanomechanical) signatures of their health and differentiating potential 80. In living cells, biological processes deeply rely on the nanomechanical properties of subcellular structures and of the cell plasma membrane or wall. By the aid of atomic force microscopy (AFM) it is now possible to gain information on the integrity and local nanomechanical properties of mammalian and microbial cellular membranes under normal or pathological conditions. The scanning probe of the AFM, usually a carbon nanotube, can sense local traits at a quasi atomic level, affording a dynamic reconstruction of mechanical, topographic, electric and thermal features, also providing important cues on optical absorption or magnetism. Importantly, AFM imaging of biological samples can be performed at sub-nanometer resolution in their natural aqueous environment, therefore allowing unprecedented characterization analyses in living cells, detecting and applying small forces with high sensitivity under physiologic conditions. This approach can also offer the chance to analyze the nanomechanics of dynamic cellular processes, including stem cell commitment and differentiation. In yeasts and bacteria, cellular activity, metabolism, growth and morphogentic changes are associated with defined nanomechanical activity, merging to the cell surface up to the generation of defined patterns of vibration 81 , 82. “Sonocytology” is the term that has been introduced to identify a novel area of inquiry based on the fact that in these small cells, after an accurate process of amplification, the pattern of frequencies corresponding to the nanomechanical motions could be transformed into audible sounds, providing a thorough acoustic representation of cellular dynamics 83.
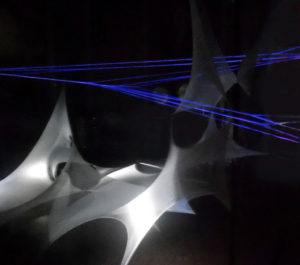
Figure 1. The early embryonic life: a Place harboring the information for our journey throughout the adulthood. The image is captured from a textile artwork (Julia von Stietencron, Art Director of VID, Visual Institute of Developmental Sciences, Bologna, Italy) representing the primordial of life as the self-assembling of forms in the presence of physical energies (i.e. electromagnetic energy and mechanical/sound vibration) symbolized by dark/light patterning and colored beams spreading throughout a network of assembling morphogenetic layers.
A movie of the artwork (10 m long, 3 m high, 3 m wide) represented as an audio/video installation, with light and sound vibration is available at: www.youtube.com/watch?v=3ftWdExdpWA

Figure 2. Tissue resident stem cells and their memory of an ancestral state The image is captured from a textile artwork (Julia von Stietencron, Art Director of VID, Visual Institute of Developmental Sciences, Bologna, Italy) symbolizing the tissue resident stem cells within their own tissue microenvironment, a niche that harbor features still resembling the early life morphogentic plains and ancestral blood vessels. The installation highlights the emergence of a new informational paradigm: our cells entail a double memory, the memory of the ongoing story of the resident tissues, and that of an ancestral state that goes back to the initial clock when embryo is fashioned from a single fertilized totipotent cell. From a singularity (single fertilized cell), the blood, vessels and all tissues take origin, and they all enwomb the original information still talking as a mutant vibration.
A movie of the artwork (8 m long, 5 m high, 5 m wide), built as an audio/video installation permeated by light beams and sounds that represent the continuous flow of information travelling in form of energy oscillation and acoustic vibration, is available at: www.youtube.com/watch?v=3ftWdExdpWA
More complex eukaryotic cells can also be investigated accordingly. For instance, stem cells directed to cardiac myocyte differentiation will begin to beat at a given moment of their commitment point. This beating motion requires a major reorganization of the cell cytoskeleton and in turn a significant change in cellular nanomechanical properties. Determination of these properties in form of measurable parameters as a function of progression throughout commitment and terminal differentiation may provide novel information on the mechanisms underlying the attainment of specific fates in individual (stem)cells 84. Other examples of cellular processes that can be measured with the AFM include activation of platelets, exocytosis, movement of cells, and cell division.
Cumulatively, these findings support the hypothesis that the chance of affording stem cell decisions is not only restricted to the chemical armamentarium but can be orchestrated by quantum nanomechanics, and that exposure to vibrational modes of sound energy may represent a worthy-of-investigation strategy for unprecedented “informative reprogramming”. If so, nanomechanics may not only harbor a “sensor” for cell fate. Rather, nanomechanical/electromagnetic patterns orchestrating stem cell commitment and differentiation may be retained and “stored” as an informative “nanomechanical signature” within the “sounds” emitted by organs and apparata even during the adult life. Such sounds may store the “informational memory” of the processes that timely afforded the functional and phenotypic specification throughout the embryonic life. Moreover, as discussed above, transferring of mechanical vibration to the subcellular environment triggers the mobilization of ionic species and the generation of ionic fluxes and induced microcurrents, ultimately ensuing in the appearance of endogenous oscillating electromagnetic fields 85 , 86. Such vibrations although derived by acoustic induced mechanical oscillations are of electromagnetic nature, no more being associated with the mechanical component of the sound, but rather with its electromagnetic counterpart, the phonon.
Based on these considerations, we are currently working on the hypothesis that cellular differentiation and reprogramming may be finely oriented by the application of physical energy through the use of localized mechanical probes and/or magnetic fields. In particular, the discovery of “audio” as a “quantum energy information (phonons)” of (stem) cell patterning may disclose the possibility of processing nanomechanics recorded from organs/tissues or cells in form of specific “sound/music” or electromagnetic patterning that can be used to direct or reprogram stem or somatic cells towards targeted differentiating outcomes 87. These strategies may represent an unprecedented tool to afford remarkable tuning of tissue/(stem) cell homeostasis, paving the way to the use of sound, music and or electromagnetic energy for optimization of (stem) cell therapy and Regenerative Medicine.
Local and Non-Local Patterning
Bearing in mind the results and considerations reported above, cells can be conceived as a fractal part of the entire organism and, to an extended view, of the entire Universe, being permeated by both local and non-local events, intimately connected by oscillatory patterns. These oscillations can avow in the form of mechanical vibrations, as well as radioelectric currents. A suitable example, although not the unique representation, of these concepts is provided by the cytoskeleton.
Mechanically, this network may be like the “elastic swing” used to synchronize the metronomes in the aforementioned experiment 88. The metronomes may be indeed represented by the overall network of signaling molecules that use the cytoskeleton as a mobile track to travel across the cells and move in and out of the nucleus, as well as the complex of chromatin regions, transcriptional regulators and transcription factors within the nucleus. It has been shown that chromatin dynamics in living cells entail oscillatory motions 89, and it has been proposed that certain chromatin regions function as synchronized oscillators, either by coupling of the electromagnetic field generated by longitudinal oscillation of nucleosomes, or by physical interaction of protein-DNA complexes 90. The density and positioning of nucleosomes in a chromosome region, the physical properties of histone octamers, as well as the protein complexes binding to the chromatin region, together determine the strength of that region. Through the mechanism described above, these chromatin regions will cluster each other if they share similar oscillation frequencies, and function as chromatin organizers to shape the higher-order chromatin structures. Theoretically, we could dissect a segment of chromatin into a number of partially entrained oscillators separated by loosely-coupled chromatin regions in which the structures are less compacted. Specific epigenetic modifications would rely on defined genome rearrangements to upstream signals, resulting in alterations of the sub-nuclear chromatin/chromosome structure. Within this context, the cytoskeleton may provide synchronization and recognition patterns among multiple oscillators. Local oscillatory changes originating at nuclear level, or in any other subcellular level, would conceivably affect the oscillatory patterns in the cytoskeleton, eliciting non-local effects by modulating the propagation of elasticity oscillations in the whole cell and the neighboring cells.
Nevertheless, the cytoskeleton may also be viewed as a generator of a dynamic oscillating electric field and radiating electromagnetic power 91. Therefore, any local change in the surface electric charge, as it occurs during protein phosphorylation or methylation in any given subcellular compartment, or following protein/DNA interaction (i.e. the formation of combinatorial DNA/transcription factor complexes) are expected to affect the electric field around oscillating microtubules. Here, non-locality may arise from the consequent modulation of electromagnetic radiation and field characteristics of the whole cellular microtubule network. To this end, intra- and inter-cellular resonance interactions between electromagnetic oscillations may be envisioned.
Intriguingly, while the non-local spreading of modulatory changes in the mechanical oscillation of the cytoskelton may be a relatively short-range phenomenon, the spreading of its modulated electric fields may go well far from the area of origin, extending to the entire organism. If we consider the propagation of the vector potential of an associated magnetic field, then the spreading of a singular cellular event may involve resonance modes even outside of the individual where a given change was generated.
Can Stem Cells be Affected by Life Rhythms and Styles?
The growing evidence linking rhythms and oscillatory patterns from a single cell to an entire organism prompts the hypothesis that a reverse flow of information may occur: if so even the life styles and rhythms adopted on personal and/or social bases may impact on the molecular and energetic pathways of our cells. Recently, to assess whether psychological well-being may affect mechanisms associated with a perspective of health advantages, Fredrickson et al 92 assessed leukocyte basal wide-ranging transcriptional profiles in 80 healthy adults who were studied for two forms, hedonic and eudaimonic, of well-being. Philosophers have long distinguished between these human attitudes, the hedonic form representing the sum of an individual’s positive affective experiences, and the eudaimonic form being amenable to a deeper mood towards a life rhythm founded upon a search for meaning and a noble purposes, far beyond a mere self-satisfaction. Both dimensions are deeply embedded in human being, the hedonic form being putatively associated to psychophysical adaptational processes, and the eudaimonic form motivating more complex social and cultural capacities. Hedonic and eudaimonic well-being showed similar affective correlates but highly divergent transcriptome profiles. Peripheral blood mononuclear cells from subjects with high levels of hedonic well-being revealed the expression of a stress-related conserved transcriptional response to adversity (CTRA), encompassing increased expression of proinflammatory genes and decreased expression of genes involved in antibody synthesis and type I IFN response 93. In contrast, high levels of eudaimonic well-being were associated with CTRA down-regulation. Promoter-based bioinformatics provided evidence for distinct patterns of transcription factor activity in structuring the observed differences in gene expression associated with eudaimonic well-being, including reduced NF-κB and AP-1 signaling, and increased Interferon Response Factor (IRF) and STAT signaling. Transcript origin analysis identified monocytes, plasmacytoid dendritic cells, and B lymphocytes as primary cellular mediators of these transcriptional patterns 94.
On the whole, these finding indicate that the human genome is extraordinarily sensitive to qualitative variations in life rhythms and choices, and raise the larger question as to whether perseverance in human attitudes may also result in different abilities of our tissue resident and circulating stem cells to express their pluripotency and rescuing potential in damaged organs, or cope with tissue or their own progression towards aging. These issues will pose intriguing challenges for the future, and may be addressed, at least in the short run, by feasible, non-invasive investigations at the level of the pool of circulating stem cells.
Conclusions
A new dynamic vision is emerging in Biology, not simply related to a generic fluctuation of molecular events, but rather fashioned on rhythms and oscillatory patterns capable to orchestrate the multifaceted world of gene and protein expression into specific trajectories, through the temporal and spatial organization of chromatin/nucleosomes oscillations, the mechanical/electromagnetic rhythms of the cytoskeleton, and the rhythmic waving of intracellular ions. These oscillatory phenomena affect crucial traits in any cell, including the stem cells that are currently becoming an essential part of the new armamentarium for approaching degenerative diseases that could not be cured even by the most advanced pharmacological or surgical treatments. It is now appearing that the interplay between complex intra- and inter-cellular circuitries must be investigated also within the attempt of deciphering mechanisms accounting for the synchronization of cellular rhythms, as they emerge from the molecular/subcellular level, up to their self-assembly at the multicellular and tissue/organ levels. Coherent with such a physical-molecular view, is the possibility to use physical energies, including electromagnetic fields and sound vibration to deeply affect the cell fate, as shown in stem cells by the optimization of their potency, or in non-stem somatic cells, by the possibility to afford their direct reprogramming toward an efficient pluripotent state. The diffusive/pervasive nature of these physical energies raises the possibility of using them to direct the multilineage potential of stem cells prior to transplantation into damaged tissues, enhancing the chance for efficacious cell therapy. On the same bases, considering the presence of resident stem cells virtually in any tissue, and the progressive decline of their rescuing ability within a damaged environment, we believe a new era is approaching in which we may talk to these cells with electromagnetic fields and sound energy, using defined amplitude, frequency and timely patterning, to reawake their rescuing potential, eventually counteracting cellular and tissue aging. This strategy may hopefully lead in a near future to develop a Regenerative Medicine without the needs for stem cell transplantation.
On the whole, new discoveries in Physics, Biology, Epigenetics, Neuroscience, and Psychology may foster Scientists, who have so far provided a fragmented picture of the living world, to develop new paradigms capable to unify the various disciplines, unraveling all the interconnections between the physical Universe and the living world, the living and the social world, the social world and knowledge. At the basis of everything in the Universe is a precise information: it forms the origin that creates the particles and all systems, including the living systems we observe. A new paradigm in Science based on information may lead to a deep transformation in the Man-Nature relationship, with unavoidable shift in medical and therapeutic approaches.
Conflict of Interests: The Authors declare that they have no conflict of interests.
References
- Peek CB, Affinati AH, Ramsey KM, et al. Circadian Clock NAD+ Cycle Drives Mitochondrial Oxidative Metabolism in Mice. Science 2013; 342(6158): 1243417. (back)
- Peek CB, Affinati AH, Ramsey KM, et al. Circadian Clock NAD+ Cycle Drives Mitochondrial Oxidative Metabolism in Mice. Science 2013; 342(6158): 1243417. (back)
- Nivala M, Ko C, Nivala M, Weiss JN, Qu Z. The Emergence of Subcellular Pacemaker Sites for Calcium Waves and Oscillations. J Physiol 2013; 591(Pt 21): 5305-5320. (back)
- Nivala M, Ko C, Nivala M, Weiss JN, Qu Z. The Emergence of Subcellular Pacemaker Sites for Calcium Waves and Oscillations. J Physiol 2013; 591(Pt 21): 5305-5320. (back)
- Nivala M, Ko C, Nivala M, Weiss JN, Qu Z. The Emergence of Subcellular Pacemaker Sites for Calcium Waves and Oscillations. J Physiol 2013; 591(Pt 21): 5305-5320. (back)
- Nivala M, Ko C, Nivala M, Weiss JN, Qu Z. The Emergence of Subcellular Pacemaker Sites for Calcium Waves and Oscillations. J Physiol 2013; 591(Pt 21): 5305-5320. (back)
- d’Eysmond T, De Simone A, Naef F. Analysis of precision in chemical oscillators: implications for circadian clocks. Phys Biol 2013; 10(5): 056005. (back)
- d’Eysmond T, De Simone A, Naef F. Analysis of precision in chemical oscillators: implications for circadian clocks. Phys Biol 2013; 10(5): 056005. (back)
- Martens EA, Thutupalli S, Fourrière A, Hallatschek O. Chimera states in mechanical oscillator networks. Proc Natl Acad Sci U S A 2013; 110: 10563-10567. (back)
- Martens EA, Thutupalli S, Fourrière A, Hallatschek O. Chimera states in mechanical oscillator networks. Proc Natl Acad Sci U S A 2013; 110: 10563-10567. (back)
- Noguchi T, Wang LL, Welsh DK. Fibroblast PER2 circadian rhythmicity depends on cell density. J Biol Rhythms 2013; 28: 183-192. (back)
- Noguchi T, Wang LL, Welsh DK. Fibroblast PER2 circadian rhythmicity depends on cell density. J Biol Rhythms 2013; 28: 183-192. (back)
- Noguchi T, Wang LL, Welsh DK. Fibroblast PER2 circadian rhythmicity depends on cell density. J Biol Rhythms 2013; 28: 183-192. (back)
- Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development 2007; 134: 1243-1251. (back)
- Masamizu Y, Ohtsuka T, Takashima Y, et al. Real-time imaging of the somite segmentation clock: revelation of unstable oscillators in the individual presomitic mesoderm cells. Proc Natl Acad Sci USA 2006; 103: 1313-1318. (back)
- Masamizu Y, Ohtsuka T, Takashima Y, et al. Real-time imaging of the somite segmentation clock: revelation of unstable oscillators in the individual presomitic mesoderm cells. Proc Natl Acad Sci USA 2006; 103: 1313-1318. (back)
- Dubrulle J, Pourquié O. Coupling segmentation to axis formation. Development 2004; 131: 5783-5793. (back)
- Palmeirim I, Henrique D, Ish-Horowicz D, Pourquié O. Avian hairy gene expression identifies a molecular clock linked to vertebrate segmentation and somitogenesis. Cell 1997; 91: 639-648. (back)
- Moloney DJ, Panin V.M, Johnston SH, et al. Fringe is a glycosyltransferase that modifies Notch. Nature 2000; 406: 369-375. (back)
- Brückner K, Perez L, Clausen H, Cohen S. Glycosyltransferase activity of Fringe modulates Notch-Delta interactions. Nature 2000; 406: 411-415. (back)
- Bessho Y, Sakata R, Komatsu S, Shiota K, Yamada S, Kageyama R. Dynamic expression and essential functions of Hes7 in somite segmentation. Genes Dev 2001; 15: 2642-2647. (back)
- Dale JK, Maroto M, Dequeant ML, Malapert P, McGrew M, Pourquié O. Periodic Notch inhibition by Lunatic Fringe underlies the chick segmentation clock. Nature 2003; 421: 275-278. (back)
- Morimoto M, Takahashi Y, Endo M, Saga Y. The Mesp2 transcription factor establishes segmental borders by suppressing Notch activity. Nature 2005; 435: 354-359. (back)
- Huppert SS, Ilagan MXG, De Strooper B, Kopan R. Analysis of Notch function in presomitic mesoderm suggests a g-secretase independent role for presenilins in somite differentiation. Dev Cell 2005; 8: 677-688. (back)
- Rajapakse I, Perlman MD, Scalzo D, Kooperberg C, Groudine M, Kosak ST. The emergence of lineage-specific chromosomal topologies from coordinate gene regulation. Proc Natl Acad Sci U S A 2009; 106: 6679-6684. (back)
- Zahanich I, Sirenko SG, Maltseva LA, et al. Rhythmic beating of stem cell-derived cardiac cells requires dynamic coupling of electrophysiology and Ca2+ cycling. J Mol Cell Cardiol 2011; 50: 66-76. (back)
- Hoey DA, Tormey S, Ramcharan S, O’Brien FJ, Jacobs CR. Primary cilia-mediated mechanotransduction in human mesenchymal stem cells. Stem Cells 2012; 30: 2561-2570. (back)
- Clement CA, Kristensen SG, Møllgård K, et al. The primary cilium coordinates early cardiogenesis and hedgehog signaling in cardiomyocyte differentiation. J Cell Sci 2009; 122(Pt 17): 3070-3382. (back)
- McMurray RJ, Wann AK, Thompson CL, Connelly JT, Knight MM. Surface topography regulates wnt signaling through control of primary cilia structure in mesenchymal stem cells. Sci Rep 2013; 3: 3545. (back)
- Bessho Y, Sakata R, Komatsu S, Shiota K, Yamada S, Kageyama R. Dynamic expression and essential functions of Hes7 in somite segmentation. Genes Dev 2001; 15: 2642-2647. (back)
- Hirata H, Yoshiura S, Ohtsuka T, et al. Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science 2002; 298: 840-843. (back)
- Bessho Y, Hirata H, Masamizu Y, Kageyama R. Periodic repression by the bHLH factor Hes7 is an essential mechanism for the somite segmentation clock. Genes Dev 2003; 17: 1451-1456. (back)
- Bessho Y, Sakata R, Komatsu S, Shiota K, Yamada S, Kageyama R. Dynamic expression and essential functions of Hes7 in somite segmentation. Genes Dev 2001; 15: 2642-2647. (back)
- Evrard YA, Lun Y, Aulehla A, Gan L, Johnson RL. Lunatic fringe is an essential mediator of somite segmentation and patterning. Nature 1998; 394: 377-381. (back)
- Zhang N, Gridley T. Defects in somite formation in lunatic fringe-deficient mice. Nature 1998; 394: 374-377. (back)
- Serth K, Schuster-Gossler K, Cordes R, Gossler A. Transcriptional oscillation of Lunatic fringe is essential for somitogenesis. Genes Dev 2003; 17: 912-925. (back)
- 25 (back)
- Mitchison HM, Schmidts M, Loges NT, et al. Mutations in axonemal dynein assembly factor DNAAF3 cause primary ciliary dyskinesia. Nat Genet 2012; 44: 381-389, S1-2. (back)
- 26 (back)
- Sládek M, Polidarová L, Nováková M, Parkanová D, Sumová A. Early chronotype and tissue-specific alterations of circadian clock function in spontaneously hypertensive rats. PLoS One 2012; 7: e46951. (back)
- Sládek M, Polidarová L, Nováková M, Parkanová D, Sumová A. Early chronotype and tissue-specific alterations of circadian clock function in spontaneously hypertensive rats. PLoS One 2012; 7: e46951. (back)
- Havelka D, Cifra M, Kučera O, Pokorný J, Vrba J. High-frequency electric field and radiation characteristics of cellular microtubule network. J Theor Biol 2011; 286: 31-40. (back)
- Zhao Y, Zhan Q. Electric fields generated by synchronized oscillations of microtubules, centrosomes and chromosomes regulate the dynamics of mitosis and meiosis. Theor Biol Med Model 2012; 9: 26. (back)
- Havelka D, Cifra M, Kučera O, Pokorný J, Vrba J. High-frequency electric field and radiation characteristics of cellular microtubule network. J Theor Biol 2011; 286: 31-40. (back)
- Havelka D, Cifra M, Kučera O, Pokorný J, Vrba J. High-frequency electric field and radiation characteristics of cellular microtubule network. J Theor Biol 2011; 286: 31-40. (back)
- 30 (back)
- Ventura C, Maioli M, Asara Y, et al. Turning on stem cell cardiogenesis with extremely low frequency magnetic fields. FASEB J 2005; 19: 155-157. (back)
- Maioli M, Rinaldi S, Santaniello S, et al. Radiofrequency energy loop primes cardiac, neuronal, and skeletal muscle differentiation in mouse embryonic stem cells: a new tool for improving tissue regeneration. Cell Transplant 2012; 21: 1225-1233. (back)
- Maioli M, Rinaldi S, Santaniello S, et al. Radiofrequency energy loop primes cardiac, neuronal, and skeletal muscle differentiation in mouse embryonic stem cells: a new tool for improving tissue regeneration. Cell Transplant 2012; 21: 1225-1233. (back)
- Maioli M, Rinaldi S, Santaniello S, et al. Radiofrequency energy loop primes cardiac, neuronal, and skeletal muscle differentiation in mouse embryonic stem cells: a new tool for improving tissue regeneration. Cell Transplant 2012; 21: 1225-1233. (back)
- Maioli M, Rinaldi S, Santaniello S, et al. Radiofrequency energy loop primes cardiac, neuronal, and skeletal muscle differentiation in mouse embryonic stem cells: a new tool for improving tissue regeneration. Cell Transplant 2012; 21: 1225-1233. (back)
- Bianchi F, Maioli M, Leonardi E, et al. A new non-enzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant 2013; 22: 2063-2067. (back)
- Maioli M, Rinaldi S, Santaniello S,et al.Radio electric asymmetric conveyed fields and human adipose-derived stem cells obtained with a non-enzymatic method and device: a novel approach to multipotency. Cell Transplant 2013; Aug 30. doi: 10.3727/096368913X672037. [Epub ahead of print] (back)
- Maioli M, Rinaldi S, Santaniello S,et al.Radio electric asymmetric conveyed fields and human adipose-derived stem cells obtained with a non-enzymatic method and device: a novel approach to multipotency. Cell Transplant 2013; Aug 30. doi: 10.3727/096368913X672037. [Epub ahead of print] (back)
- Maioli M, Rinaldi S, Santaniello S,et al.Radio electric asymmetric conveyed fields and human adipose-derived stem cells obtained with a non-enzymatic method and device: a novel approach to multipotency. Cell Transplant 2013; Aug 30. doi: 10.3727/096368913X672037. [Epub ahead of print] (back)
- Baal N, Reisinger K, Jahr H, et al. Expression of transcription factor Oct-4 and other embryonic genes in CD133 positive cells from human umbilical cord blood. Thromb Haemost 2004; 92: 767-775. (back)
- Goodell MA. Stem-cell “plasticity”: befuddled by the muddle. Curr Opin Hematol 2003; 10: 208-213. (back)
- Lang KC, Lin IH, Teng HF, et al. Simultaneous overexpression of Oct4 and Nanog abrogates terminal myogenesis. Am J Physiol Cell Physiol 2009; 297: C43-54. (back)
- Park SB, Seo KW, So AY, et al. SOX2 has a crucial role in the lineage determination and proliferation of mesenchymal stem cells through Dickkopf-1 and c-MYC. Cell Death Differ 2012; 19: 534-545. (back)
- 38 (back)
- Maioli M, Rinaldi S, Santaniello S, et al. Radio electric conveyed fields directly reprogram human dermal skin fibroblasts toward cardiac, neuronal, and skeletal muscle-like lineages. Cell Transplant 2013; 22: 1227-1235. (back)
- Maioli M, Rinaldi S, Santaniello S, et al. Radio electric conveyed fields directly reprogram human dermal skin fibroblasts toward cardiac, neuronal, and skeletal muscle-like lineages. Cell Transplant 2013; 22: 1227-1235. (back)
- Rinaldi S, Maioli M, Santaniello S, et al. Regenerative treatment using a radioelectric asymmetric conveyor as a novel tool in antiaging medicine: an in vitro beta-galactosidase study. Clin Interv Aging 2012; 7: 191-194. (back)
- Maioli M, Rinaldi S, Santaniello S, et al. Anti-senescence efficacy of radio-electric asymmetric conveyer technology. Age (Dordr) 2014; 36(1): 9-20. (back)
- Maioli M, Rinaldi S, Santaniello S, et al. Anti-senescence efficacy of radio-electric asymmetric conveyer technology. Age (Dordr) 2014; 36(1): 9-20. (back)
- Sawada R, Ito T, Tsuchiya T. Changes in expression of genes related to cell proliferation in human mesenchymal stem cells during in vitro culture in comparison with cancer cells. J Artif Organs 2006; 9: 179-184. (back)
- 42 (back)
- Collodel G, Fioravanti A, Pascarelli NA, et al. Effects of regenerative radioelectric asymmetric conveyer treatment on human normal and osteoarthritic chondrocytes exposed to IL-1β. A biochemical and morphological study. Clin Interv Aging 2013; 8: 309-316. (back)
- Maioli M, Rinaldi S, Santaniello S, et al. Anti-senescence efficacy of radio-electric asymmetric conveyer technology. Age (Dordr) 2014; 36(1): 9-20. (back)
- Collodel G, Fioravanti A, Pascarelli NA, et al. Effects of regenerative radioelectric asymmetric conveyer treatment on human normal and osteoarthritic chondrocytes exposed to IL-1β. A biochemical and morphological study. Clin Interv Aging 2013; 8: 309-316. (back)
- Huang CH, Chen MH, Young TH, Jeng JH, Chen YJ. Interactive effects of mechanical stretching and extracellular matrix proteins on initiating osteogenic differentiation of human mesenchymal stem cells. J Cell Biochem. 2009; 108: 1263-1273. (back)
- Doyle AM, Nerem RM, Ahsan T. Human mesenchymal stem cells form multicellular structures in response to applied cyclic strain. Ann Biomed Eng 2009; 37: 783-793. (back)
- Wolf CB, Mohammad RK. Mechano-transduction and its role in stem cell biology. Baharvand H, ed. Trends in stem cell biology and technology. Totowa, NJ: Humana Press, 2009; pp. 389-403. (back)
- Higuchi T, Anton M, Dumler K, et al. Combined reporter gene PET and iron oxide MRI for monitoring survival and localization of transplanted cells in the rat heart. J Nucl Med 2009; 50: 1088-1094. (back)
- Vajkoczy P, Blum S, Lamparter M, et al. Multistep nature of microvascular recruitment of ex vivo-expanded embryonic endothelial progenitor cells during tumor angiogenesis. J Exp Med 2003; 197: 1755-1765. (back)
- Aicher A, Heeschen C, Sasaki K, Urbich C, Zeiher AM, Dimmeler S. Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: a new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation 2006; 114: 2823-2830. (back)
- Lionetti V, Cantoni S, Cavallini C, et al. Hyaluronan mixed esters of butyric and retinoic acid affording myocardial survival and repair without stem cell transplantation. J Biol Chem 2010; 285: 9949-9961. (back)
- Hu BS, Landeen LK, Aroonsakool N, Giles WR. An analysis of the effects of stretch on IGF-I secretion from rat ventricular fibroblasts. Am J Physiol Heart Circ Physiol 2007; 293: H677-683. (back)
- Abrams GA, Goodman SL, Nealey PF, Franco M, Murphy CJ. Nanoscale topography of the basement membrane underlying the corneal epithelium of the rhesus macaque. Cell Tissue Res 2000; 299: 39-46. (back)
- INTERNATIONAL PATENT: Gimzewski JK, Pelling A, and Ventura C. International Publication Number WO 2008/105919 A2, International Publication Date 4 September 2008. Title: Nanomechanical Characterization of Cellular Activity. (back)
- INTERNATIONAL PATENT: Gimzewski JK, Pelling A, and Ventura C. International Publication Number WO 2008/105919 A2, International Publication Date 4 September 2008. Title: Nanomechanical Characterization of Cellular Activity. (back)
- Pelling AE, Sehati S, Gralla EB, Valentine JS, Gimzewski JK. Local nanomechanical motion of the cell wall of Saccharomyces cerevisiae. Science 2004; 305: 1147-1150. (back)
- INTERNATIONAL PATENT: Gimzewski JK, Pelling A, and Ventura C. International Publication Number WO 2008/105919 A2, International Publication Date 4 September 2008. Title: Nanomechanical Characterization of Cellular Activity. (back)
- INTERNATIONAL PATENT: Gimzewski JK, Pelling A, and Ventura C. International Publication Number WO 2008/105919 A2, International Publication Date 4 September 2008. Title: Nanomechanical Characterization of Cellular Activity. (back)
- Havelka D, Cifra M, Kučera O, Pokorný J, Vrba J. High-frequency electric field and radiation characteristics of cellular microtubule network. J Theor Biol 2011; 286: 31-40. (back)
- Zhao Y, Zhan Q. Electric fields generated by synchronized oscillations of microtubules, centrosomes and chromosomes regulate the dynamics of mitosis and meiosis. Theor Biol Med Model 2012; 9: 26. (back)
- INTERNATIONAL PATENT: Gimzewski JK, Pelling A, and Ventura C. International Publication Number WO 2008/105919 A2, International Publication Date 4 September 2008. Title: Nanomechanical Characterization of Cellular Activity. (back)
- Martens EA, Thutupalli S, Fourrière A, Hallatschek O. Chimera states in mechanical oscillator networks. Proc Natl Acad Sci U S A 2013; 110: 10563-10567. (back)
- Pliss A, Malyavantham KS, Bhattacharya S, Berezney R. Chromatin dynamics in living cells: identification of oscillatory motion. J Cell Physiol 2013; 228: 609-616. (back)
- Zhao Y, Zhan Q. Electric oscillation and coupling of chromatin regulate chromosome packaging and transcription in eukaryotic cells. Theor Biol Med Model 2012; 9: 27. (back)
- Havelka D, Cifra M, Kučera O, Pokorný J, Vrba J. High-frequency electric field and radiation characteristics of cellular microtubule network. J Theor Biol 2011; 286: 31-40. (back)
- Fredrickson BL, Grewen KM, Coffey KA, et al. A functional genomic perspective on human well-being. Proc Natl Acad Sci U S A 2013; 110: 13684-13689. (back)
- Fredrickson BL, Grewen KM, Coffey KA, et al. A functional genomic perspective on human well-being. Proc Natl Acad Sci U S A 2013; 110: 13684-13689. (back)
- Fredrickson BL, Grewen KM, Coffey KA, et al. A functional genomic perspective on human well-being. Proc Natl Acad Sci U S A 2013; 110: 13684-13689. (back)
To cite this article
Fashioning Cellular Rhythms with Magnetic Energy and Sound Vibration: a New Perspective for Regenerative Medicine
CellR4 2014; 2 (2): e839
Publication History
Published online: 31 Mar 2014

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.